Cleaning up our act: Materialising new ideas for a better planet
INNOVATION | NNL | SOLVENT AND EFFLUENT TREATMENT | UNIVERSITIES AND ACADEMIAAs the need for nuclear energy expands to meet Net Zero, so does the need to manage stored nuclear materials. However, this also raises an opportunity to do things differently. AFCP’s Advanced Solvent and Effluent Treatment team is exploring novel decontamination materials that are both sustainably sourced and industry optimised. Antony Nearchou, Research Fellow at the University of Birmingham, discusses how sharing strengths has enabled this impactful innovation.

Challenge
Effective effluent treatment with sustainability of supply
Sustainable thinking means considering the long-term effects of our present actions. As the role of nuclear grows to meet the UK’s clean energy demand, this involves creating innovative solutions around one of our sector’s largest challenges: the decontamination and disposal of radioactive waste. The Advanced Fuel Cycle Programme’s (AFCP) Advanced Solvent and Effluent Treatment project is addressing this, identifying how to best minimise the environmental, economic and sociocultural impacts of managing used nuclear materials.
Once nuclear fuel has been used, it is cooled in large water storage ponds. However, many of the radioactive fission products are soluble, meaning they can easily dissolve and contaminate the water. Two of the most problematic products are cesium (Cs-137) and strontium (Sr-90) cations, which are highly soluble and produce most of the remaining medium-lived radioactivity in the storage ponds. The challenge here is that these cations are incredibly mobile in water, so if they leach into the environment, they can contaminate the water table. Therefore, to prevent leaching it is important that these radioactive cations are removed and immobilised in a form that can be safely disposed.
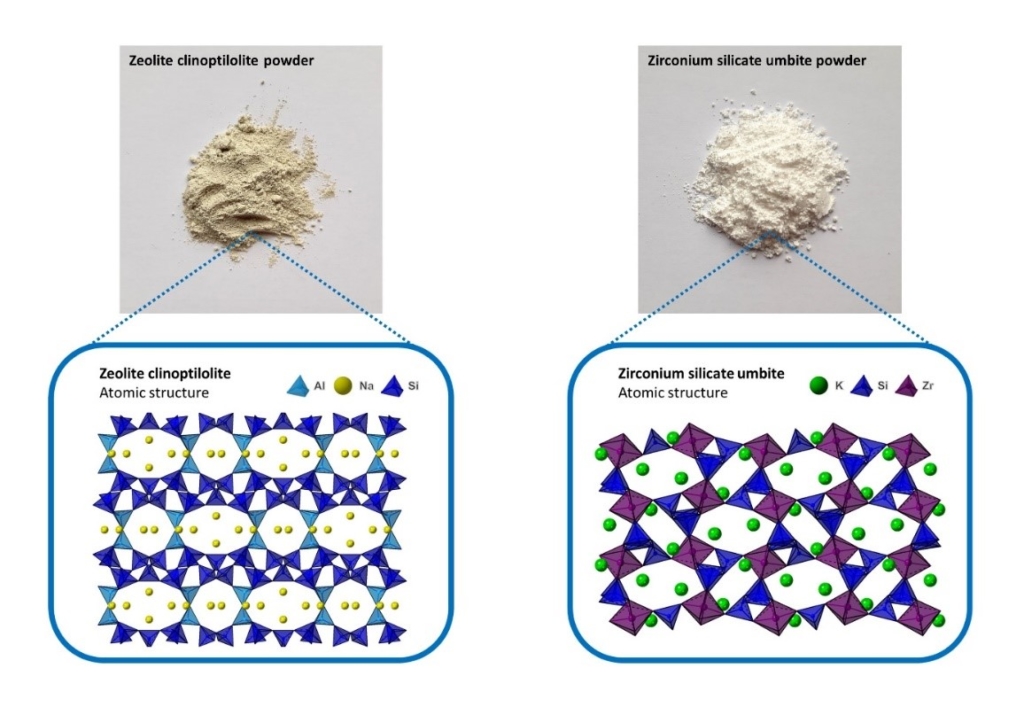
Currently, the nuclear industry uses porous silicate minerals known as zeolites to capture these radioactive cations from storage ponds. The process is known as ion exchange, where the cations already present in the zeolite are mobile and can swap with the cations in solution. The zeolites chosen are capable of selectively targeting and capturing Cs-137 and Sr-90 from the storage ponds. Recent laboratory-scale research conducted by the National Nuclear Laboratory (NNL) and University of Birmingham has highlighted how zeolite clinoptilolite used in industry is very effective at removing Cs-137 and Sr-90 from a variety of solution conditions seen in different nuclear waste streams. Furthermore, the use of zeolites is highly appealing due to their low cost, high capacity for cations and their capability of being converted into denser ceramic phases where the radioactive cations are trapped within the material, minimising the risk of leaching.
One of the problems facing this process is the supply of the zeolites used, such as clinoptilolite. These materials are mined in countries across the world, presenting geopolitical issues with supply. Similar zeolites mined from different locations display variations in performance. Additionally, these earth materials are finite. As we move toward more sustainable operations as an industry, we must consider alternative ideas to preserve limited natural resources. Therefore, there is a need to find new successor materials that we can use to continue the decontamination of storage ponds and radioactive effluent.
That said, identifying new materials comes with further technical challenges. Storage ponds contain a variety of dissolved species with much greater concentrations than Cs-137 and Sr-90, meaning the new materials need to be selective in the presence of any competitors. Another factor to consider is that depending on the industrial process, the pH of waste solutions can vary. Consequently, successor materials must be resistant to degradation and maintain capture of Cs-137 and Sr-90 in highly caustic solutions.

Solution
Industry-ready innovation with promising potential
Through AFCP’s Advanced Solvent and Effluent Treatment project, we have been able to combine our materials expertise in academia with the NNL team’s knowledge of chemical waste streams. Working with NNL, we were able to undertake research into how these new materials could be deployed in an industrial setting. Our team in Materials Chemistry at the University of Birmingham has not previously considered this aspect in detail, meaning this project presented an opportunity to explore new ground.
Our AFCP team at the University of Birmingham has identified several prospective materials that industry could use to succeed zeolites for radioactive waste decontamination. These materials are porous tin and zirconium silicates, which can be prepared in a laboratory with the possibility of being produced at a large scale to meet industry’s demand.
The materials successfully overcome the technical challenges under consideration. For example, they display promising selective capture of Cs cations in the presence of competing cations that are typically seen in waste streams. The materials maintain uptake of Cs cations in both acidic and alkaline solutions, remaining stable under varying conditions.
A common problem in research is that materials of interest are prepared as loose powders. Pelletising the loose powder is a requisite for industry, as it enables materials to be handled and incorporating into flow systems and treatment plants more easily. We have successfully prepared the tin and zirconium silicates into bound pellets. Our results show that the pellets maintain the Cs capture and caustic stability properties of the loose powders. Additionally, preliminary data indicates that after Cs uptake, these materials can easily be converted into denser ceramic phases where the cations are trapped in the structure. Collectively, these early results present these silicates as promising materials for deployment in industry.
AFCP has presented new opportunities for us and the capability to study these materials using NNL’s specialist measurement techniques that are not available at the University of Birmingham. Our new collaboration with NNL will allow an assessment of how the materials perform in capturing Cs-137 from radioactive solutions under real-world conditions.
Impact
Powerful partnerships for a sustainable future
The innovation of these materials is an asset to the UK nuclear sector, strengthening the arsenal of methods available for decontamination of radioactive waste. This will provide greater flexibility for the sector to adapt the decontamination process and use the best material for specific waste streams. In addition, our research into the deployment and potential waste forms of these materials aids the nuclear sector in planning how they will be disposed of in a geological disposal facility (GDF).
As we continue to adopt more sustainable fuel cycles in the UK, these materials have promise to reduce our sector’s environmental impact from mining through to disposal. With potential to scale up this concept, the work may support deployment opportunities for UK-made materials while reducing our reliance on newly mined zeolite. This not only minimises our consumption of finite resources, but also ensures a more ethical and available supply of materials through the future.
AFCP has established and fostered a new collaboration between Birmingham and NNL, which we expect to be enduring. This has enabled professional development in advancing working relationships with industry and combining expertise for a common goal. Both our teams are also eager to take the work forward for publication, expand the academic profile of the participants and widen the work’s influence across the scientific community.
In addition to professional development, our AFCP partnership with NNL has allowed us to advance our research capability. Our team at the University of Birmingham has benefited by gaining access to cutting-edge NNL facilities to undertake specialist testing with radioactive solutions. Furthermore, the project has allowed us to improve our research skills and methods so that they better align with industry. Our research can therefore be directly translated to the sector – such as through pelletising the material into a more functional form – and hence provide more impactful innovation.
AFCP is part of the Department for Business, Energy and Industrial Strategy’s (BEIS) £505m Energy Innovation Programme.
